At Micro Measurement Laboratories, we excel in particle analysis, offering comprehensive support for particulate matter testing from start to finish. Whether it’s method development, verification, lot release testing, or root cause investigation, our team of experts is equipped to handle your most challenging material characterization needs. With a wide array of advanced instruments and technologies at our disposal, we’re ready to deliver precise and reliable results.
Particulate Matter Testing
USP General Testing Chapters such as:
- USP <729>, Globule Size Distribution in Lipid Injectable Emulsions
- USP <787>, Subvisible Particulate Matter in Therapeutic Protein Injections
- USP <788>, Particulate Matter in Injections. Method I, Light Obscuration and Method II, Microscopic Particle Count Test
- USP <789>, Particulate Matter in Ophthalmic Solutions. Light Obscuration and Microscopic Particle Count Test
- ISO 8871-3, Elastomeric Parts for Parenterals and for Devices for Pharmaceutical Use
- ISO 8536, Infusion Equipment for Medical Use
- ISO 14708, Implants for Surgery– Active implantable medical devices
- BS EN 45502-2-1:2003, Active implantable medical devices Particular requirements for active in plantable medical devices intended to treat bradyarrhythmia (cardiac pacemakers)
- ASTM International Testing Procedures
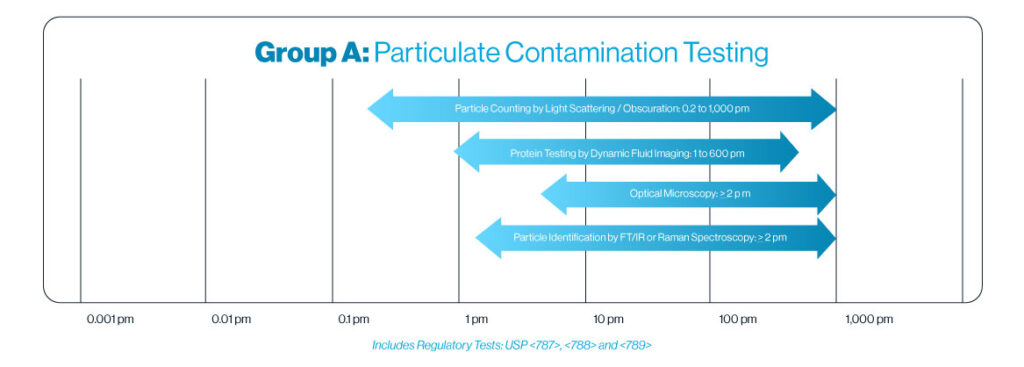
Particle Counting by Light Obscuration and Light Scattering
Particle counting by light obscuration is a precise analytical method used to measure and size particles in a liquid suspension. Particles pass between a laser light source and a detector. As each particle blocks or scatters light, the detector measures the reduction of light intensity and processes the data to quantify particles and determine their size.
Our volumetric liquid particle counters measure the entire liquid stream to provide quantities and sizes ranging from 0.2 μm to 1,000 μm in up to 1,024 channels.
Optical Microscopy
Particle counting using optical microscopy involves visually examining particulate matter collected on a filtration membrane to identify and quantify individual particles. This method allows for direct observation of particle size, shape and distribution at working ranges from 2 µm and greater.
Particle Counting by Flow Imaging
This technique utilizes digital imaging to measure particles in a flowing fluid stream. This method allows for precise analysis of particle size, particle shape (morphology), shape analysis, grayscale or color attributes, and statistical population distribution. With a working range starting from 2 µm, this technique is particularly effective for measuring biologics and proteins. By capturing detailed images, we can provide comprehensive data on particle characteristics, ensuring accurate and reliable results for your analytical needs.

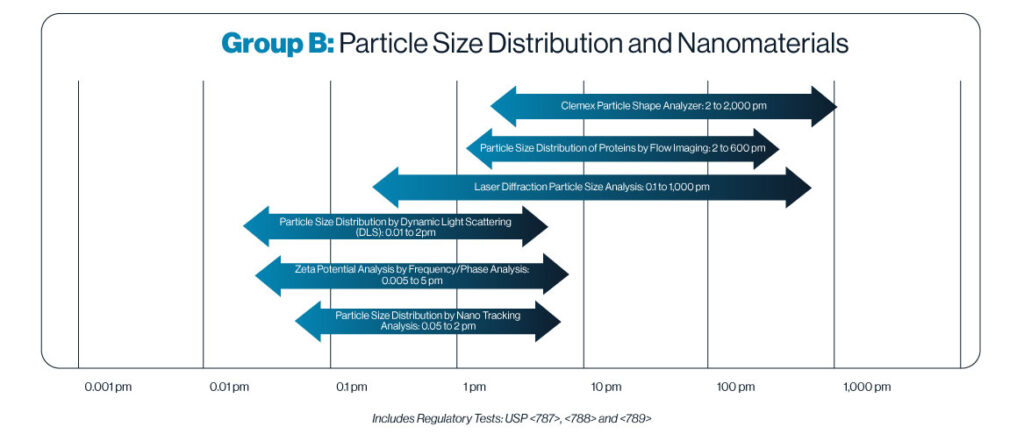
Particle Size Distribution
Nano Tracking Analysis (NTA) – A technique used to visualize and analyze nanoparticles in liquid suspensions. NTA uses a laser to illuminate nanoparticles in a liquid sample. The scattered light from these particles is captured by a microscope and recorded by a camera, allowing real-time visualization. By analyzing the movement of particles, NTA is able to calculate particle sizes ranging from 50 nm to 2 µm by using the Stokes-Einstein equation.
Laser Diffraction Spectroscopy – A technique used to measure the size distribution of particles in a sample. Using a laser beam and photodetectors, a laser beam passes through the sample, scattering light in various directions. Large particles scatter light at small angles, while small particles scatter at larger angles. The scattering pattern is then analyzed using the Mie theory to determine the particle size distribution. At MML, we are able to measure sizes ranging from 20 nm to 4 mm.
Dynamic Light Scattering – also known as photon correlation spectroscopy (PCS), or quasi-elastic light scattering (QLS). DLS measures the browning motion of particles in a liquid. The random movement of particles causes fluctuations in the intensity of scattered light, which are analyzed using the Stokes-Einstein equation to convert the diffusion coefficient into particle size, from 10 nm to 2 µm.
Additional Services:
Feasibility Evaluation Testing & Method Development

Unlock critical insights during formulation and product development with our cutting-edge orthogonal methods. By leveraging state-of-the-art instruments and techniques, we can identify particulate matter, extractables, fines, aggregate issues, and changes in particle size distribution—without the high costs of early-stage method validation or development. Trust MML’s expertise to deliver valuable information from feasibility tests using proven methods.
Method Validation

Partner with us to determine the optimal combination of tests for validating your sample material. Our protocols are built on ICH, FDA, and regulatory requirements, ensuring they meet the stringent standards of regulatory authorities. With experience in instruments, methods, and a variety of issues, we benefit from testing a wide variety of similar materials, which is advantageous even for CROs.
Method Transfer or Verification

Transferring methods between facilities can be challenging, even with the same manufacturer’s instruments. With 30 years of experience in particle instrumentation and method development, we provide deep insights into system and location differences, facilitating faster method transfer or verification. We also lead and participate in reference studies and round-robin comparisons for thorough verification.


